Zeta potential on the surface of a fiber
4.Measurement of surface zeta potential of fibrous material
It was very difficult to measure zeta potential of fibrous substances until now because the fibers themselves absorb water and water leakage occurs even if fibrous or filamentous substances are placed in a flat surface cell. However, it was found that if the fibrous material is surrounded by an insulating film as shown in Fig. 3, water leakage does not occur, and measurement can be performed easily. Using this sample fixation method, the surface zeta potential of a filamentous sample was attempted to measure.
(1)Measurement conditions
pH dependence of surface zeta potential of botanical fiber cotton and animal fiber silk threads were measured with the flat surface cell by changing pH of monitor particle solution from 2 to 6. As mentioned in the part 2, polystyrene latex, whose particle diameter is about 500 nm and which is coated with hydroxypropyl cellulose to reduce the zeta potential to almost zero, was used as standard monitor particles. They were dispersed in 10 mM NaCl solution so that they became slightly cloudy. 0.1 M to 1 M HCl was used to adjust the pH of the solution.
Electrophoretic mobility of the surface of the sample was obtained by measuring at 7 points inside the glass cell and analyzing electroosmotic flow with Mori-Okamoto’s equation. Surface zeta potential was calculated from obtained electrophoretic mobility with Smoluchowski's6) equation.
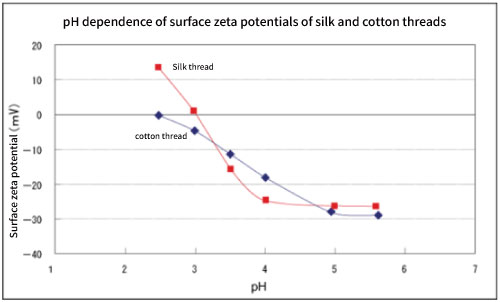
Figure 4. pH dependence of surface zeta potentials of silk and cotton threads.
(2) Measurement results and discussion
Fig. 4 shows the pH dependence of the surface zeta potentials of cotton and silk threads. Cotton threads have negative zeta potential at any pH and reached to the saturation near pH 6. And the pH corresponding to the half of saturated zeta potential is 3.5 to 4, which is almost the same as the pKa (pH 3 to 4) of carboxyl group.
Since cotton thread is a negative fiber and has an acidic group (carboxyl group), it seems that it has a pH dependence that reflects it. By utilizing this, it may be possible to qualitatively know the acidic dissociation groups of unknown negative fibers 7). On the other hand, the zeta potential of silk thread which is amphoteric fiber has a positive value on the acidic side, and the isoelectric point where the charge becomes zero shows near pH 3. This is a reasonable result because it has an acidic group (carboxyl group) and a basic group (amino group), and the isoelectric point (near pH 3) is in good agreement with the literature values 8).
| References | 6) | Smoluchowski,M.:Z.phys.Chem.92, 129 (1918) |
| 7) | Toshiro Suzawa: Industrial chemistry, 62,232,1231 (1959); 63,1069,2205 (1961); 64,573 (1961); Dyes and Chemicals, 8,543 (1963); Surface, 2,14 (1964) | |
| 8) | Toshiro Suzawa: Dyeing Industry, 12, 198 (1964) |


 Close
Close

