Application:various examples of zeta potential measurement
1.pH titration of alumina
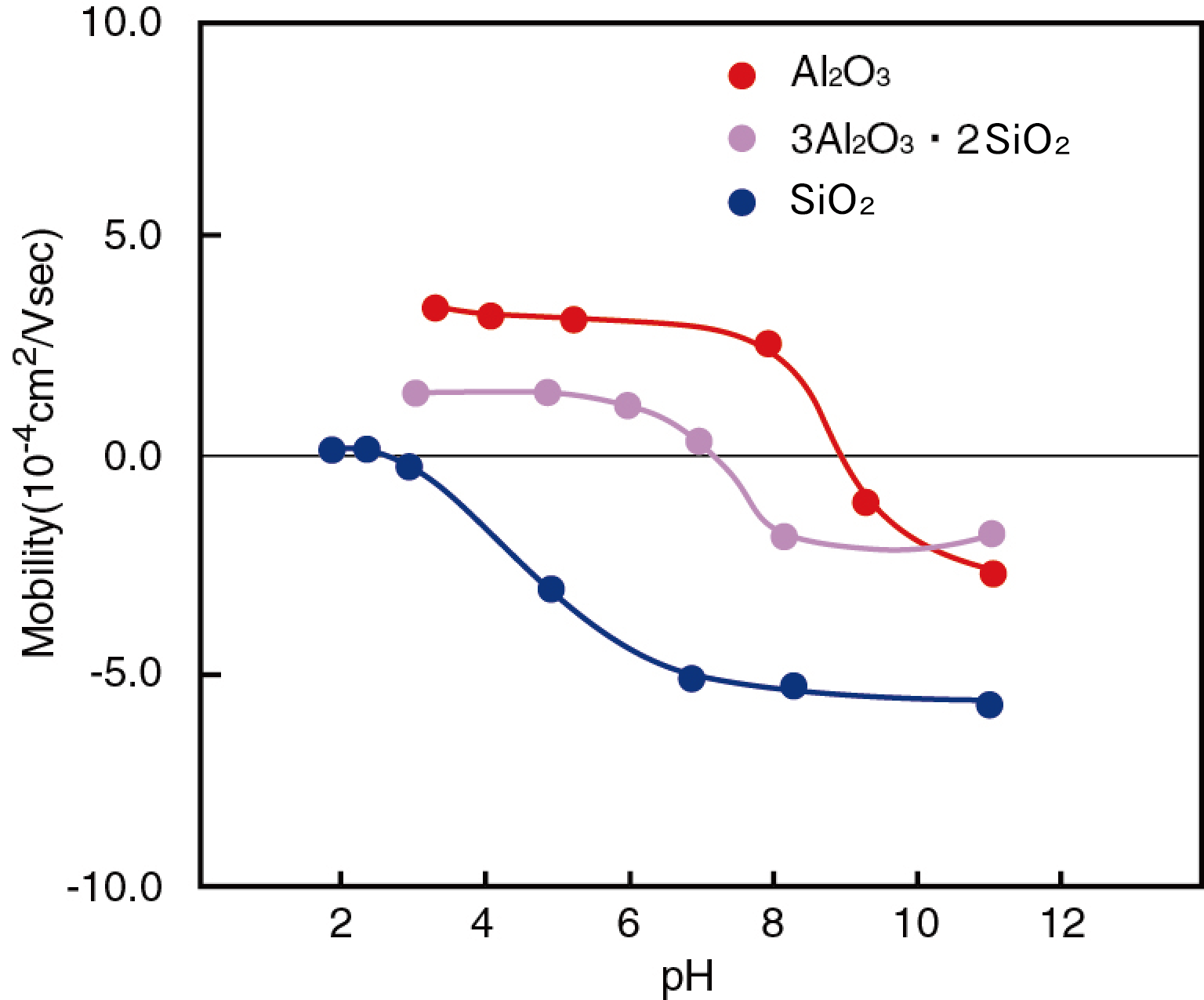
For inorganic oxide particles, the zeta potential changes significantly when the pH value of the solution changes. They have the isoelectric point where the surface potential becomes zero at a specific pH and any interfacial electrophoretic phenomena such as electrophoresis does not show. Since the electrostatic repulsive force disappears at this isoelectric point, particles is easy to aggregate. To stabilize the dispersion system, it is important to keep the pH of system as far away from the isoelectric point as possible and increase the absolute value of zeta potential.


 Close
Close

