Application:various examples of zeta potential measurement
4.Zeta potential measurement in non-aqueous solvent
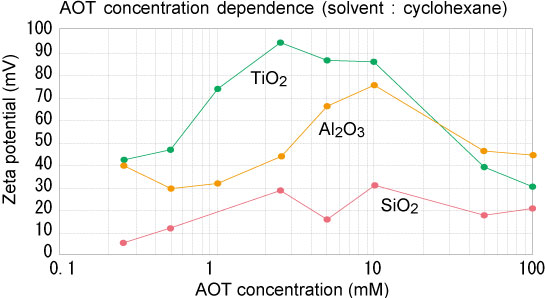
In the zeta potential measurement in a non-aqueous solvent (especially a low dielectric constant solvent), the applied voltage, the temperature uniformity inside the cell, the cell material / structure, etc. are important compared to the measurement in the aqueous solvent. Therefore, it is possible to measure stably with a specially designed cell for a low dielectric constant solvent.
Here, the effect of dispersant in the alumina-cyclohexane-AOT system was measured. When plotting the zeta potential (calculated from the mobility by Hückel's equation) with respect to the AOT addition concentration, the zeta potential changes depending on the AOT addition concentration. When 10 mM is added, the zeta potential is the largest, which indicates the excellent dispersibility.


 Close
Close

